Which Of The Following Cells Are Responsible For Repair In The Dermis?
Introduction
Granulation tissue is an important component in the wound healing process. Wounds can heal by primary intention (wound edges approximate easily) and secondary intention (wounds edges do not approximate). Granulation tissue matrix will fill wounds that heal by second intention. This tissue type is also present in chronic wounds that have many different causes. This article will discuss the mechanism of wound healing and repair, the role that granulation tissue plays in that process, the pathophysiology resulting in chronic wounds and persistent granulation tissue, and the clinical significance of granulation tissue.
Cellular
Granulation tissue is considered a contractile organ, characterized histologically by the presence and proliferation of fibroblasts, keratinocytes, endothelial cells, new thin-walled capillaries, and inflammatory cell infiltration of the extracellular matrix. The formation of granulation tissue is complex and requires an intricate interplay between the cell types at the wound site.
Fibroblasts
This cell type is responsible for the formation of the granulation tissue extracellular matrix (ECM). The ECM initially consists of type III collagen, which is a rapidly produced and a weaker form of collagen that is ultimately replaced by the stronger type I collagen at the end of wound healing and scar formation. TGF-β is a growth factor produced by both fibroblasts and keratinocytes during the wound healing process and has been shown to induce granulation tissue formation and the differentiation of myofibroblasts.
Keratinocytes
The keratinocytes are responsible for re-epithelialization of the epidermis after injury. The ability of the keratinocytes to differentiate and proliferate depends on the stem cell population at the wound site and stimulation by various cytokines and growth factors (epidermal growth factor, TGF-α & KGF: keratinocyte growth factor). The cell to cell binding between keratinocytes is looser during the wound healing process and becomes stronger by its completion. Immediately after injury, the keratinocytes adjacent to the wound undergo an activation process that alters gene expression and promotes their migration over the bed of the wound.
In an autocrine and paracrine fashion, keratinocytes produce signals that regulate more keratinocyte activation and stimulate other cell types (fibroblasts) during wound closure. IL-1 increases keratinocyte migration and proliferation, activate nearby fibroblasts, increases KGF (keratinocyte growth factor). TNF-α acts similarly to IL-1 and activates the FGF (fibroblast growth factor) pathway. TGF-β, produced by keratinocytes and fibroblasts, acts to revert keratinocytes to their basal cell phenotype. This signaling process is deficient in venous ulcers and contributes to non-healing.
Endothelial Cells
The endothelial cells are responsible for revascularization/angiogenesis at the site of the wound. Initially quiescent, the resident endothelial cells become activated by several angiogenic factors, including fibroblast growth factor, vascular endothelial growth factor (VEGF), platelet-derived growth factor (PDGF), angiogenin, and transforming growth factors α & β (TGFα & TGFβ). Once activated, the endothelial cells undergo four events in the formation of new blood vessels:
-
Protease production: breakdown of ECM
-
Chemotaxis
-
Proliferation
-
Remodeling and differentiation
Angiogenesis requires that the endothelial cells grow quickly into the new tissue from the older intact blood vessels that branch out and form anastomoses with other vessels and restoring blood flow. This process is important for the restoration of the flow of nutrients and oxygen to the site, removing waste, and transportation of leukocytes to the site. Without this, granulation tissue formation would be stunted and wound healing prolonged.
Myofibroblasts
Myofibroblasts are responsible for the contractile process in wound closure. When granulation tissue is forming, fibroblasts slowly modulate into myofibroblasts, characterized by bundles of actin microfilaments along their cell's plasma membrane. Prostaglandins, bradykinins, epinephrine, and norepinephrine modulate the function of contraction by the myofibroblasts. Eventually, the actin binds to the extracellular component fibronectin, attaches to the collagen fibers, retracts, and draws collagen fibers toward it. The mass effect causes wound contraction and closure.
Immune Cells
Neutrophils (first to the wound) and macrophages are the primary immune cells at the site of wounds that aid in wound healing and protect against invading pathogens. Both cell types are phagocytic, meaning that they engulf and ingest pathogens, debris, and damaged tissue, allowing for protection and healing.[1][2][3]
Function
Granulation tissue is a type of new connective tissue, and microscopic blood vessels have three main functions.
-
Immune: Protects the wound surface from microbial invasion and further injury.
-
Proliferative: Fills the wound from its base with new tissue and vasculature.
-
Temporary plug: Replaces necrotic tissue until replacement by scar tissue.[1][2]
Mechanism
The process of wound healing is vastly different when comparing fetuses and adults. Fetal wound healing typically has minimal to no scarring, little to no inflammation (thought to be due to a lack of myofibroblasts), rapid epithelialization, high proportions of type III collagen, fast fibroblast migration, and less degranulation by fibroblasts.
Under normal adult conditions, wound healing and tissue repair occur in four stages:
-
Hemostasis(Scab formation)
-
Inflammatory Stage(Inflammation and edema formation)
-
Proliferative Stage(Granulation tissue formation)
-
Remodeling Stage (Scar formation)
Hemostasis
During this stage of wound healing, the blood vessels at the site of injury contract, and the blood that leaked out through the wounds begin to coagulate and scab over. The coagulation process results from the aggregation of thrombocytes, forming a fibrin network. The fibrin network is the basis for a clot and serves as a temporary matrix for migrating cells. The thrombocytes release cytokines and growth factors that serve as pro-inflammatory signals to attract the immune response to the site of the wound.
Inflammatory Stage
This stage begins when neutrophils begin to arrive at the wound site. Neutrophils arrive at the wound within a few minutes and continue to accumulate for several days. The neutrophils' role is to entrap the microorganisms present at the wound site from the beginning of the injury. The neutrophils also amplify the pro-inflammatory response and release their own markers.
Monocytes arrive at the site of injury within two days, where they differentiate into macrophages. The role of macrophages is to phagocytose debris, pathogens, and neutrophils while also producing chemical mediators (TGF-beta and VEGF) that serve to recruit fibroblasts and endothelial cells that mark the next stage of healing. During this stage, the site of injury becomes erythematous and edematous. This stage takes about 72 hours to complete.
Proliferative Stage
During this stage of healing, re-epithelialization and the replacement of the clot by granulation tissue occurs simultaneously. This stage is highly cellular with the primary cell types, including fibroblasts, keratinocytes, and endothelial cells. Fibroblasts function to synthesize collagen and the extracellular matrix. The granulation tissue itself is made up of extracellular matrix, proteoglycans, hyaluronic acid, collagen, and elastin. Cytokines and growth factors, interleukins, and angiogenesis factors are active during this time, which can continue anywhere from days to weeks under normal circumstances. Granulation tissue grows from the base of the wound and can typically fill any size wound. Any errors in the formation of granulation tissue can result in chronic wound formation. Foreign bodies at the site of a wound can also result in persistent granulation tissue and poor wound healing with excess macrophages, fibroblasts, and capillaries reacting around the foreign material.
Remodeling Stage
The last stage of wound healing involves apoptosis and the production of new cells. The apoptosis and degradation of the extracellular matrix and immature type III collagen and the ultimate formation of mature type I collagen are critical to scar formation, integrity, and strength. This stage can continue from months to years. If there are errors during this phase, there may be excessive wound healing leading to hypertrophic or keloidal scarring or a chronic wound resulting in persistent granulation tissue.
Healthy granulation tissue appears pink to red due to new capillary formation, is soft to the touch, moist appears "bumpy," and typically painless. Unhealthy granulation tissue is darker red, bleeds easily with minimal contact, painful, and maybe covered by shiny white or yellow fibrous tissue that is avascular, impeding healing. When granulation tissue appears "unhealthy," an infectious process or poor healing should be suspected. Wound culture and appropriate treatment according to the culture results should be completed as soon as possible. Once the infection is ruled out or cleared, some chronic wounds may respond to simple cautery with silver nitrate or with topical steroid preparations, while those with the overlying fibrous tissue will require debridement with a curette or scalpel to allow for healing.[1][2][3][4][5][6][7]
Pathophysiology
Wound healing can become prolonged under several circumstances. The pathophysiology of wound healing, resulting in excess and unhealthy granulation tissue and chronic wounds are complex. The causes may include infection at the site of the wound resulting in excessive recruitment of inflammatory cells. Infections result in increased release of reactive oxygen species that are damaging to the tissue and can also result in the formation of biofilms, which contribute to poor wound healing and are caused by bacterial toxins. Infections can lead to further complications, including abscess, cellulitis, osteomyelitis, and loss of limbs.
Necrosis, poor perfusion, and poor nutrition/metabolism due to insufficient blood supply due to the trauma itself, poor angiogenesis, and underlying conditions (diabetes and vascular insufficiency) are also sources of chronic wounds and persistent granulation tissue.[1][2][3][4][8]
Clinical Significance
Granulation tissue may persist under several circumstances resulting from underlying conditions and/or errors in the stages of wound healing (errors in the proliferative phase and remodeling phase).[1][2][3][4][6][9][8]
Possible causes of prolonged wound healing include:
-
Diabetes mellitus
-
Rheumatoid arthritis
-
Vascular or arterial insufficiency
-
Zinc deficiency
-
Low human growth hormone levels
-
Older age
-
Obesity
-
Trauma
-
Excess moisture
-
Tissue hypoxia
-
Medications (steroids, NSAIDs, chemotherapy)
-
Alcoholism
-
Tobacco abuse
Possible causes of Hypergranulation (excess granulation tissue)
-
Wound infection
-
Excess inflammation
-
Foreign body/material
-
Physical irritation/friction
Granulation tissue can also present in:
-
Pyogenic granuloma
-
Pulp polyps
Review Questions
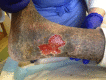
Figure
Venous stasis ulceration in classic lower medial leg (gaiter region) location. Note typical features of shallow wound base, irregular borders, healthy red granulation tissue, and surround lipodermatosclerosis. Contributed by Mark A. Dreyer, DPM, FACFAS (more...)

Figure
Pyogenic granuloma H/E 10x. The lobular morphology is changed by secondary ulceration and inflammatory changes. In this picture the epidermis is ulcerated and neutrophil are widely occupying the lesion. An epidermal collarette is present over a true granulation (more...)

Figure
Granulation tissue, H/E, 20x. In this image true granulation tissue is formed in a pre-existing pyogenic granuloma/angioma. The lobular morphology of pyogenic granuloma can be obscured by ulceration and inflammatory changes. Contributed by Fabiola Farci, (more...)
References
- 1.
-
Demidova-Rice TN, Hamblin MR, Herman IM. Acute and impaired wound healing: pathophysiology and current methods for drug delivery, part 1: normal and chronic wounds: biology, causes, and approaches to care. Adv Skin Wound Care. 2012 Jul;25(7):304-14. [PMC free article: PMC3428147] [PubMed: 22713781]
- 2.
-
Guo S, Dipietro LA. Factors affecting wound healing. J Dent Res. 2010 Mar;89(3):219-29. [PMC free article: PMC2903966] [PubMed: 20139336]
- 3.
-
Pastar I, Stojadinovic O, Yin NC, Ramirez H, Nusbaum AG, Sawaya A, Patel SB, Khalid L, Isseroff RR, Tomic-Canic M. Epithelialization in Wound Healing: A Comprehensive Review. Adv Wound Care (New Rochelle). 2014 Jul 01;3(7):445-464. [PMC free article: PMC4086220] [PubMed: 25032064]
- 4.
-
Jaeger M, Harats M, Kornhaber R, Aviv U, Zerach A, Haik J. Treatment of hypergranulation tissue in burn wounds with topical steroid dressings: a case series. Int Med Case Rep J. 2016;9:241-5. [PMC free article: PMC4986970] [PubMed: 27570466]
- 5.
-
Dreifke MB, Jayasuriya AA, Jayasuriya AC. Current wound healing procedures and potential care. Mater Sci Eng C Mater Biol Appl. 2015 Mar;48:651-62. [PMC free article: PMC4443476] [PubMed: 25579968]
- 6.
-
Hiremath R, Reddy H, Ibrahim J, Haritha CH, Shah RS. Soft Tissue Foreign Body: Utility of High Resolution Ultrasonography. J Clin Diagn Res. 2017 Jul;11(7):TC14-TC16. [PMC free article: PMC5583796] [PubMed: 28892999]
- 7.
-
Mo Y, Sarojini H, Wan R, Zhang Q, Wang J, Eichenberger S, Kotwal GJ, Chien S. Intracellular ATP Delivery Causes Rapid Tissue Regeneration via Upregulation of Cytokines, Chemokines, and Stem Cells. Front Pharmacol. 2019;10:1502. [PMC free article: PMC6976531] [PubMed: 32009945]
- 8.
-
Li X, Kim J, Wu J, Ahamed AI, Wang Y, Martins-Green M. N-Acetyl-cysteine and Mechanisms Involved in Resolution of Chronic Wound Biofilm. J Diabetes Res. 2020;2020:9589507. [PMC free article: PMC7007959] [PubMed: 32083136]
- 9.
-
Livingstone D, Alghonaim Y, Jowett N, Sela E, Mlynarek A, Forghani R. Silver nitrate mimicking a foreign body in the pharyngeal mucosal space. World J Radiol. 2015 May 28;7(5):100-3. [PMC free article: PMC4444602] [PubMed: 26029352]
Which Of The Following Cells Are Responsible For Repair In The Dermis?
Source: https://www.ncbi.nlm.nih.gov/books/NBK554402/
Posted by: kleinyourbithes.blogspot.com

0 Response to "Which Of The Following Cells Are Responsible For Repair In The Dermis?"
Post a Comment